Daniel Scott | Health Science Correspondent
Animal testing, albeit with scandal, is mandatory in the vast majority of cases of drug development. Those Hamilton-based will be familiar with activist campaigns against animal testing on campus at Lincoln Gate, the TBSI’s front door has been smashed on various occasions and, through the grapevine, the latter’s loading bay was developed to prevent public interjection during animal delivery.
The use of animals in scientific research has been something unavoidable. In vitro (in glass) is usually the first test completed in drug discovery or toxicological analysis, where the drug is tested for its effects on cell lines which generally include a receptor for the drug/toxin to act on. This determines how much of the drug is needed to purport effect (potency) as well as the duration of action. Animal testing (in vivo) involves challenging the drug to be delivered to the interested site of action – as opposed to being dropped on top of specialised cells in a petri dish, the drug must pass the physiological barriers of fatty membrane permeation, dodging enzymes in the cell or liver which can inactivate the drug by metabolism, or how rapid excretion may be. In vivo also gives the drug a chance to demonstrate any toxic effect which has not been accounted for on other targets.
The drug then moves on to Phase I – where the drug is given in augmenting increments to a healthy volunteer to determine its metabolic and excretory patterns, which as I am sure you can appreciate, is a different ballpark to a mouse or a rabbit.
In 2006, a UK clinical trial involved a massive inflammatory reaction and multi organ dysfunction in some of the six volunteers following administration of an immune altering biotech treatment intended for leukaemia.
While this has been all well and good for past drug developments of small molecules, the pharmaceutical market has been flooded by multiple options for statins, oral contraceptives and antidepressants. Little incentive is due to massive competition, tied in with the decade of research and billion dollar price tag at a minimum to get a drug to market. While gene therapies and stem cells are something massively funded and researched, the current focus availability lies with biotechnological products.
However in 2006, a UK clinical trial involved a massive inflammatory reaction and multi organ dysfunction in some of the six volunteers following administration of an immune altering biotech treatment intended for leukaemia. The dose given was the same as significantly less than what was administered to a mouse and been recognised as safe. All six men fought for their lives in hospital, luckily survived but lifetime impacts followed, as well as the fear of further consequences in the future. The cause of all this? One hypothesis is the animals used in testing had been bred in a relatively sheltered environment in a lab for their lifetime, thus their exposure to viruses, bacteria and allergens was limited. In the human’s case, the immune system falsified what was thought to have been a previously exposed to antigen which led to a massive inflammatory attack. Although this has been deemed an extremely rare case with regards to dire jeopardy in volunteer safety, as medical interventions become more specific and intimate with biological pathways with added expense, a more specific and economical alternative is needed.
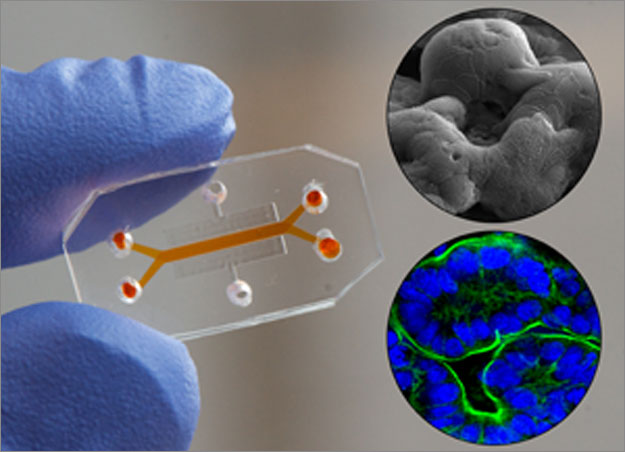
A more hands-on and physiological approach is offered by The Wyss Institute, based in Harvard University, whom have drafted “organs-on-chips”. The “Lung on a Chip” was the first project undertaken, where a thin tube was formed, divided up by lung cells to form two discrete compartments, one for air supply, the other for blood supply.
A move from animal testing has been advocated in the last few decades by both lobbying parties, academia and researchers alike. One particular area where this movement has been accepted in the eyes of the legislation is regarding testing of cosmetic products. In the past ten years, there has been deadlines set via the EU Cosmetic Directive on testing finished products down to separate ingredients. The FDA has also given no indication for a mandatory animal testing at any point for the licensing of cosmetics in the US. Many in vitro models have successfully shown a quasi physiological representation of skin, by indicating potential irritation reactions, permeation of components as well as toxicity studies . Limits are finally set by the directive to prove that any ingredient used does not pass to the dermal layer or must have a previously established toxicity profile.
A more hands-on and physiological approach is offered by The Wyss Institute, based in Harvard University, whom have drafted “organs-on-chips”. It’s pitch has received vast media attention following their Lead Senior Scientist of Biomimetic Microsystems, Dr. Geraldine Hamilton’s TED talk. The organ on a chip project is a multidisciplinary research project, involving computer scientists, biologists and engineers alike. These miniature devices the size of memory sticks encompass cell culture lines in order to create customisable quasi-physiological conditions depending on what is added to the chip. The “Lung on a Chip” was the first project undertaken, where a thin tube was formed, divided up by lung cells to form two discrete compartments, one for air supply, the other for blood supply.
Common pathologies were stimulated successfully, such as a simple inflammation sequence following bacteria introduced to the air supply and white blood cells added into the blood. Since the development of the chip, ten different organs-on-chips have been synthesised, with a potential to couple the different organs together to create a “human-on-chip” in the future. The project also has other precious applications. In May of this year, it was announced in Nature Magazine that the first “Barth disease-on-chip” was developed, a model for a rare cardiovascular disorder generally found in males to which there is no known treatment. By transferring a patient’s skin cells carrying the defunct gene that caused the illness to the heart-on-chip platform, the cells were ployed into differentiating to cardiac-like cells due to the surrounding environment. Other diseases such as cystic fibrosis, asthma and inflammatory bowel syndrome have been highlighted for development with this technology. It gives a manageable, affordable insight into the effects of a disease in a human model: something rare today.
An even further bonus is the fact that a miniature “live” model of the disease is created. The project also has a scope for toxicological analysis, especially in the case of exploring environmental toxins by untrained individuals.
This research and innovation based product has become a startup, with Sony DADC joining forces with the Wyss institute in order to scale organs-on-chips into a reproducible, affordable lab must-have. Although this may seem a huge divide from this branch of the tech giant’s CD and DVD production, the flexible polymer which the organ-on-chip is supported on allows a symbiotic business deal, with an automated production line possible for this product as well as breathing life into the firm’s dwindling share price.
In spite of high failure rates, misinterpreted correlations between animal models and ludicrously high costs of drug development, the organs-on-chips technology offers something which might raise the thermostat of the “ice-age” being felt in drug development today.